Histopathological and real–time PCR analysis of alterations in the rat brain within the Glycerol–induced Crush Syndrome
Abstract
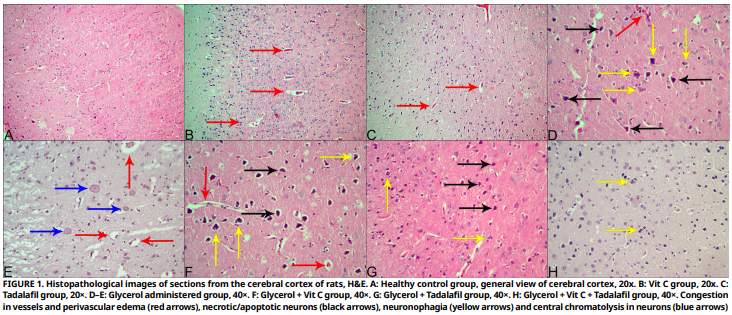
This study attempted to examine the neuroprotective benefits of vitamin C and tadalafil in an experimental crush syndrome model induced by intramuscular glycerol injection in rats, using histopathological and real–time polymerase chain reaction. 50 females Wistar albino rats were used in the study. The animals were divided into 7 groups: healthy control (n: 6), vitamin C (n: 6), tadalafil (n: 6), glycerol administered (n: 8), glycerol + vitamin C (n: 8), glycerol + tadalafil (n: 8), and glycerol + vitamin C + tadalafil (n: 8). After the 6–day experimental study, the animals were anesthetized and euthanized, and necropsies were performed. For histopathological and real–time polymerase chain reaction analyses brain tissues were fixed in 1% formaldehyde solution. Sections of 5 µm thickness were obtained from tissues processed by routine histological procedures and stained with hematoxylin and eosin. Microscopic examination revealed that glycerol administration caused neuronal necrosis, neuronophagia, gliosis, edema, congestion, endothelial cell damage, and mononuclear cell infiltration. Brain Natriuretic Peptide, Heat Shock Protein 70 and Hypoxia–Inducible Factor 1–alpha expression levels were measured in real–time polymerase chain reaction analysis. Glycerol administration caused increases in Brain Natriuretic Peptide and Heat Shock Protein 70 levels in the brain, while it did not cause any significant changes in Hypoxia–Inducible Factor 1–alpha levels. Decreases in Brain Natriuretic Peptide and Heat Shock Protein 70 expression levels were detected in the glycerol + Vit C, glycerol + tadalafil, and glycerol + Vit C + tadalafil groups. According to the research results, it is thought that the combined application of Vit C, tadalafil and Vit C + tadalafil can provide protection against oxidative and inflammatory stress against brain damage that may occur after glycerol–induced crush syndrome.
Downloads
References
Desai SN, Desai PV. Aspartate aminotransferase and alanine aminotransferase activities of rat brain during crush syndrome. Neurosci. Lett. [Internet]. 2008; 447(1):58–61. doi: https://doi.org/ckfpdq DOI: https://doi.org/10.1016/j.neulet.2008.09.043
Cohen BH, Gaspar MP, Daniels AH, Akelman E, Kane PM. Multifocal neuropathy: expanding the scope of double crush syndrome. J. Hand Surg. [Internet]. 2016; 41(12):1171–1175. doi: https://doi.org/f9dmc3 DOI: https://doi.org/10.1016/j.jhsa.2016.09.009
Desai Shanti N, Desai PV. The study of Na+, K+–ATPase activity of rat brain during Crush syndrome. Neurochem. Res. [Internet]. 2007; 32(11):1843–1848. doi: https://doi.org/fhbjvs DOI: https://doi.org/10.1007/s11064-007-9370-5
Martinez T, Laemmel E, Bergis B, Rustin M, Huertas A, Harrois A, Libert N. Beyond acute kidney injury, traumatic rhabdomyolysis associated to hemorrhagic shock is responsible for multiple–organ failure in a rat model. Shock 2025; 2025:10.1097. doi: https://doi.org/qk5v DOI: https://doi.org/10.1097/SHK.0000000000002668
Özkıdık M, Gökce Mİ, Yaman Ö. Efficacy of tadalafil treatment on erectile dysfunction in patients under dutasteride treatment: A prospective non–randomized comparative study. Turk. J. Urol. [Internet]. 2018; 44(4):294–297. doi: https://doi.org/qk5w DOI: https://doi.org/10.5152/tud.2018.46666
Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther. Clin. Risk Manag. [Internet]. 2008; 4(6):1315–1329. doi: https://doi.org/g78kfq DOI: https://doi.org/10.2147/TCRM.S3336
Nilsson D, Chess–Williams R, Sellers D. Phosphodiesterase-5 inhibitors tadalafil and sildenafil potentiate nitrergic–nerve mediated relaxations in the bladder vasculature. Eur. J. Pharmacol. [Internet]. 2023; 960:176152. doi: https://doi.org/qk5z DOI: https://doi.org/10.1016/j.ejphar.2023.176152
Şahin Ç, Yıldırım N, Hortu İ, Akdemir A, Özşener S, Yiğittürk G, Erbaş O. Tadalafil attenuates ischemic damage as well as reperfusion injury in the rat ovary. J. Turk. Ger. Gynecol. Assoc. [Internet]. 2020; 21(1):35–40. doi: https://doi.org/gm9nrv DOI: https://doi.org/10.4274/jtgga.galenos.2019.2018.0121
Seftel AD. Phosphodiesterase type 5 inhibitor differentiation based on selectivity, pharmacokinetic, and efficacy profiles. Clin. Cardiol. [Internet]. 2004; 27(S1):14–19. doi: https://doi.org/fqmkvr DOI: https://doi.org/10.1002/clc.4960271305
Porst H, Padma–Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology [Internet]. 2003; 62(1):121–125. doi: https://doi.org/c6x22p DOI: https://doi.org/10.1016/S0090-4295(03)00359-5
Said R, Mahmoud O, Mohammed A, Abd El Maksoud H. Biochemical role of Tadalafil in experimental brain impairment activities in mice. Benha Vet. Med. J. [Internet]. 2024; 46(1):50–53. doi: https://doi.org/qk52 DOI: https://doi.org/10.21608/bvmj.2024.264516.1774
Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS, Shin MC, Cho HJ, Kim SC, Kim SH, Kim KH, Shin DH, Kim CJ. Tadalafil improves short–term memory by suppressing ischemia– induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol. Biochem. Behav. [Internet]. 2009; 91(4):629–635. doi: https://doi.org/cwkn22 DOI: https://doi.org/10.1016/j.pbb.2008.10.009
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. [Internet]. 2003; 22(1):18–35. doi: https://doi.org/ggrks6 DOI: https://doi.org/10.1080/07315724.2003.10719272
Kaźmierczak–Barańska J, Boguszewska K, Adamus–Grabicka A, Karwowski BT. Two faces of vitamin C–antioxidative and pro–oxidative agent. Nutrients [Internet]. 2020; 12(5):1501. doi: https://doi.org/gmw3bp DOI: https://doi.org/10.3390/nu12051501
Lúcio M, Nunes C, Gaspar D, Ferreira H, Lima JL, Reis S. Antioxidant activity of vitamin E and Trolox: understanding of the factors that govern lipid peroxidation studies in vitro. Food Biophys. [Internet]. 2009; 4(4):312–320. doi: https://doi.org/dzwfms DOI: https://doi.org/10.1007/s11483-009-9129-4
Kangisser L, Tan E, Bellomo R, Deane AM, Plummer MP. Neuroprotective properties of vitamin C: a scoping review of pre–clinical and clinical studies. J. Neurotrauma [Internet]. 2021; 38(16):2194–2205. doi: https://doi.org/qk53 DOI: https://doi.org/10.1089/neu.2020.7443
Ahmad A, Shah SA, Badshah H, Kim M J, Ali T, Yoon GH, H Kim T, B Abid N, Rehman SU, Khan S, O Kim M. Neuroprotection by vitamin C against ethanol–induced neuroinflammation associated neurodegeneration in developing rat brain. CNS. Neurol. Disord. Drug Targets [Internet]. 2016; 15(3):360–370. doi: https://doi.org/f8g2bz DOI: https://doi.org/10.2174/1871527315666151110130139
Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature [Internet]. 1988; 332:78–81. doi: https://doi.org/b4d9pk DOI: https://doi.org/10.1038/332078a0
Hosoda K, Nakao K, Mukoyama M, Saito Y, Jougasaki M, Shirakami G, Suga G, Ogawa Y, Yasue H, Imura H. Expression of brain natriuretic peptide gene in human heart. Production in the ventricle. Hypertension [Internet]. 1991; 17(6_ pt_2):1152–1155. doi: https://doi.org/qk54 DOI: https://doi.org/10.1161/01.HYP.17.6.1152
Alcidi G, Goffredo G, Correale M, Brunetti ND, Iacoviello M. Brain natriuretic peptide biomarkers in current clinical and therapeutic scenarios of heart failure. J. Clin. Med. [Internet]. 2022;11(11):3192. doi: https://doi.org/g83qkw DOI: https://doi.org/10.3390/jcm11113192
Harpaz D, Seet RC, Marks RS, Tok AI. B–type natriuretic peptide as a significant brain biomarker for stroke triaging using a bedside point–of–care monitoring biosensor. Biosensors [Internet]. 2020; 10(9):107. doi: https://doi.org/qk6h DOI: https://doi.org/10.3390/bios10090107
Katoh C, Osanai T, Tomita H, Okumura K. Brain natriuretic peptide is released from human astrocytoma cell line U373MG under hypoxia: a possible role in anti–apoptosis. J. Endocrinol. [Internet]. 2011; 208(1):51–57. doi: https://doi.org/bz44g9 DOI: https://doi.org/10.1677/JOE-10-0230
Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia–inducible factor 1. Kidney Int. [Internet]. 1997; 51(2):553–555. doi: https://doi.org/bghh74 DOI: https://doi.org/10.1038/ki.1997.77
Cramer T, Johnson RS. A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell cycle [Internet]. 2003; 2(3):191–192. doi: https://doi.org/btj42h DOI: https://doi.org/10.4161/cc.2.3.402
Adams J, Difazio L, Rolandelli R, Luján j, Haskó Gy, Csóka B, Selmeczy Zs, Németh Z. HIF–1: a key mediator in hypoxia (Review). Acta Physiol. Hung. [Internet]. 2009; 96(1):19–28. doi: https://doi.org/fv5g7f DOI: https://doi.org/10.1556/APhysiol.96.2009.1.2
Schumacker PT. Hypoxia–inducible factor–1 (HIF–1). Crit. Care Med. [Internet]. 2005; 33(12):S423-S425. doi: https://doi.org/ffg57v DOI: https://doi.org/10.1097/01.CCM.0000191716.38566.E0
Howell NJ, Tennant DA. The role of HIFs in ischemia– reperfusion injury. Hypoxia [Internet]. 2014; 2:107–115. doi: https://doi.org/qk6k DOI: https://doi.org/10.2147/HP.S49720
Fulda S, Debatin K–M. HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell cycle [Internet]. 2007; 6(7):790–792. doi: https://doi.org/fn3285 DOI: https://doi.org/10.4161/cc.6.7.4084
Balamurugan K. HIF–1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer [Internet]. 2016; 138(5):1058–1066. doi: https://doi.org/ggwwk2 DOI: https://doi.org/10.1002/ijc.29519
Hagymasi AT, Dempsey JP, Srivastava PK. Heat–shock proteins. Curr. Protoc. [Internet]. 2022; 2(11):e592. doi: https://doi.org/qk6m DOI: https://doi.org/10.1002/cpz1.592
Janowska MK, Baughman HE, Woods CN, Klevit RE. Mechanisms of small heat shock proteins. Cold Spring Harb. Perspect. Biol. [Internet]. 2019; 11(10):a034025. doi: https://doi.org/qk6n DOI: https://doi.org/10.1101/cshperspect.a034025
Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. [Internet]. 2010; 92(2):184–211. doi: https://doi.org/cngkw5 DOI: https://doi.org/10.1016/j.pneurobio.2010.05.002
Liu Y, Yu M, Chen L, Liu J, Li X, Zhang C, Xiang X, Li X, Lv Q. Systemic review of animal models used in the study of crush syndrome. Shock [Internet]. 2022; 57(4):469–478. doi: https://doi.org/g5pq9x DOI: https://doi.org/10.1097/SHK.0000000000001911
Lv Q, Long M, Wang X, Shi J, Wang P, Guo X, Song J, Midgley AC, Fan H, Hou S. The role of alpha–1–acid glycoprotein in the diagnosis and treatment of crush syndrome–induced acute kidney injury. Shock [Internet]. 2021; 56(6):1028–1039. doi: https://doi.org/qk6q DOI: https://doi.org/10.1097/SHK.0000000000001839
Karamalakova YD, Georgieva ED, Petkova–Parlapanska KV, Slavova VB, Ivanova KV, Ivanov VA, Nikolova GD. Silybum marianum reduces acute kidneys injury by modifying biochemical changes and oxidative stress levels in glycerol– induced Crush Syndrome. Bulg. Chem. Commun. [Internet]. 2024; 56(D2):109–115. doi: https://doi.org/qk6r DOI: https://doi.org/10.34049/bcc.56.D.S2P53
Koç A. Crush syndrome: a review of current knowledge and treatment strategies. J. Compr. Surg. [Internet]. 2023; 1(1):7–10. doi: https://doi.org/qk6s DOI: https://doi.org/10.51271/JOCS-0002
Selvi MH. The use of statistics in veterinary sciences and the test methods used. Res. Pract. Vet. Anim. Sci. [Internet]. 2024; 1(1):43–50. doi: https://doi.org/n4v9
Gu H, Yang M, Zhao X, Zhao B, Sun X, Gao X. Pretreatment with hydrogen–rich saline reduces the damage caused by glycerol–induced rhabdomyolysis and acute kidney injury in rats. J. Surg. Res. [Internet]. 2014; 188(1):243–249. doi: https://doi.org/f5w768 DOI: https://doi.org/10.1016/j.jss.2013.12.007
Nara A, Yajima D, Nagasawa S, Abe H, Hoshioka Y, Iwase H. Evaluations of lipid peroxidation and inflammation in short– term glycerol–induced acute kidney injury in rats. Clin. Exp. Pharmacol. Physiol. [Internet]. 2016; 43(11):1080–1086. doi: https://doi.org/f9cgbh DOI: https://doi.org/10.1111/1440-1681.12633
Singh AP, Singh AJ, Singh N. Pharmacological investigations of Punica granatum in glycerol–induced acute renal failure in rats. Indian J. Pharmacol. [Internet]. 2011; 43(5):551–556. doi: https://doi.org/dchmpm DOI: https://doi.org/10.4103/0253-7613.84971
Aydogdu N, Atmaca G, Yalcin O, Taskiran R, Tastekin E, Kaymak K. Protective effects of l–carnitine on myoglobinuric acute renal failure in rats. Clin. Exp. Pharmacol. Physiol. [Internet]. 2006; 33(1–2):119–124. doi: https://doi.org/d2znfh DOI: https://doi.org/10.1111/j.1440-1681.2006.04336.x
Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. [Internet]. 1996; 49(2):314–326. doi: https://doi.org/d2hnht DOI: https://doi.org/10.1038/ki.1996.48
Gong T, Liu L, Jiang W, Zhou R. DAMP–sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. [Internet]. 2020; 20(2):95–112. doi: https://doi.org/gg264m DOI: https://doi.org/10.1038/s41577-019-0215-7
Butler CA, Popescu AS, Kitchener EJ, Allendorf DH, Puigdellívol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. [Internet]. 2021; 158(3):621–639. doi: https://doi.org/gh4h4f DOI: https://doi.org/10.1111/jnc.15327
Yu F, Wang Y, Stetler AR, Leak RK, Hu X, Chen J. Phagocytic microglia and macrophages in brain injury and repair. CNS. Neurosci. Ther. [Internet]. 2022; 28(9):1279–1293. doi: https://doi.org/grshnb DOI: https://doi.org/10.1111/cns.13899
Perkins G, Valberg SJ, Madigan JM, Carlson GP, Jones SL. Electrolyte disturbances in foals with severe rhabdomyolysis. J. Vet. Intern. Med. [Internet]. 1998; 12(3):173–177. doi: https://doi.org/btdjbd DOI: https://doi.org/10.1111/j.1939-1676.1998.tb02114.x
Guerrero–Hue M, García–Caballero C, Palomino–Antolín A, Rubio–Navarro A, Vázquez–Carballo C, Herencia C, Martín– Sanchez D, Farré–Alins V, Egea J, Cannata P, Praga M, Ortiz A, Egido J, Sanz AB, Moreno JA. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis–mediated cell death. FASEB J. [Internet]. 2019; 33(8):8961–8975. doi: https://doi.org/ghc46h DOI: https://doi.org/10.1096/fj.201900077R
Safari S, Aghili SH, Shahlaee MA, Kerachi AJ, Ranjbar MF. Incidence of electrolyte imbalances following traumatic rhabdomyolysis: A systematic review and meta–analysis. Cureus [Internet]. 2024; 16(4):e59333. doi: https://doi.org/g8zrrq
Sánchez–Moreno C, Paniagua M, Madrid A, Martín A. Protective effect of vitamin C against the ethanol mediated toxic effects on human brain glial cells. J. Nutr. Biochem. [Internet]. 2003; 14(10):606–613. doi: https://doi.org/fcjwjs DOI: https://doi.org/10.1016/j.jnutbio.2003.07.003
Kumar RR, Singh L, Thakur A, Singh S, Kumar B. Role of vitamins in neurodegenerative diseases: a review. CNS. Neurol. Disord. Drug Targets [Internet]. 2022; 21(9):766–773. doi: https://doi.org/g9q6st DOI: https://doi.org/10.2174/1871527320666211119122150
Yeşilyurt M, Yüksel S. Basınç yaralanmalı hastaların tedavisinde beslenmenin etkisi [The effect of nutrition in the treatment of pressure injury patients]. Genel. Sağlık Bilim. Derg. [J. Gen. Health Sci]. [Internet]. 2020; 2(3):200–207. Turkish. doi: https://doi.org/qk6t DOI: https://doi.org/10.51123/jgehes.2020.10
Gulati P, Singh N. Tadalafil enhances the neuroprotective effects of ischemic postconditioning in mice, probably in a nitric oxide associated manner. Canadian J. Physiol. Pharmacol. [Internet]. 2014; 92(5):418–426. https://doi.org/f524nq DOI: https://doi.org/10.1139/cjpp-2013-0428
Okada A, Kashima Y, Tomita T, Takeuchi T, Aizawa K, Takahashi M, Ikeda U. Characterization of cardiac oxidative stress levels in patients with atrial fibrillation. Heart Vessels [Internet]. 2016; 31(1):80–87. doi: https://doi.org/f75b49 DOI: https://doi.org/10.1007/s00380-014-0582-8
Chang P, Zhang X, Zhang J, Wang J, Wang X, Li M, Wang R, Yu J, Fu F. BNP protects against diabetic cardiomyopathy by promoting Opa1-mediated mitochondrial fusion via activating the PKG–STAT3 pathway. Redox Biol. [Internet]. 2023; 62:102702. doi: https://doi.org/qk6v DOI: https://doi.org/10.1016/j.redox.2023.102702
Van–Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF–induced lethal inflammatory shock. Immunity [Internet]. 2002; 16(5):685–695. doi: https://doi.org/ccq65s DOI: https://doi.org/10.1016/S1074-7613(02)00310-2
Belenichev IF, Aliyeva OG, Popazova OO, Bukhtiyarova NV. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: The prospect of using HSP70 modulators. Front. Cell. Neurosci. [Internet]. 2023; 17:1131683. doi: https://doi.org/qk6w DOI: https://doi.org/10.3389/fncel.2023.1131683