Efficacy of water–based propolis in preventing intra–abdominal adhesions in an experimental rat model
Abstract
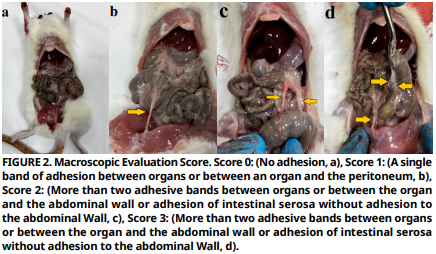
Intra–abdominal adhesions are one of the most common postoperative complications following surgical procedures. Globally, it is among the leading causes of hospital readmissions, chronic pain, and serious conditions such as infertility in both human and veterinary medicine. The risk of development is particularly high after pelvic and abdominal surgeries. Despite this common condition, there is still no effective and reliable treatment method to prevent adhesions. This study aims to evaluate the potential effect of propolis (both oral and intra–abdominal) with its anti–inflammatory and antioxidant properties in the prevention of intra–abdominal adhesions. The rats used in the study were divided into five: Healthy Control Group, Control Group , Sham Group , Oral Propolis Group , and Intra–abdominal Propolis Group . After the intra–abdominal adhesion model was established in rats, excluding the Healthy Control Group and Sham Group, rats in the Oral Propolis Group were administered 75 mg·kg-1 of propolis orally once daily for 20 days, while rats in the Intra–abdominal Propolis Group received a single dose of 75 mg·kg-1 of propolis intra–abdominally. Results showed that the Oral Propolis Group and Intra–abdominal Propolis Group were statistically significantly lower than the Control Group in terms of macroscopic adhesion scores. Although there was no statistically significant difference when the Oral Propolis Group and Intra–abdominal Propolis Group were compared with the Control Group in terms of fibrosis, it was observed that the Intra–abdominal Propolis Group was statistically significantly lower than the Control Group in terms of inflammation severity. Biochemical analyses, it was revealed that the oxidative stress parameters of rats in both oral and intra–abdominal propolis application were statistically different from the Control Group and that these two were close to the Healthy Control Group in terms of oxidative stress. As a result, it was concluded that both intra– abdominal and oral propolis applications prevent the formation of intra–abdominal adhesions and can be used for prophylactic purposes after abdominal and pelvic operations.
Downloads
References
Kiliç N. The effect of Aloe vera gel on experimentally induced peritoneal adhesions in rats. Revue Méd. Vét. [Internet]. 2005 [cited July 21, 2025]; 156(7):409–413. Available in :https://goo.su/YWt4eik
Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J. Gastroenterol. [Internet]. 2011; 17(41):4545–4553. doi: https://doi.org/bthgrq DOI: https://doi.org/10.3748/wjg.v17.i41.4545
Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: A review of the literature. Am. J. Surg. [Internet]. 2011; 201:111–121. doi: https://doi.org/db96kb DOI: https://doi.org/10.1016/j.amjsurg.2010.02.008
Köm M. Tavşanlarda Postoperatif İntraabdominal Adezyonların Önlenmesinde Hyaluronik asit/Karboksimetilselüloz Bariyerlerin Etkinliği [The efficacy of hyaluronic acid/ carboxymethylcellulose barriers on the prevention of postoperative ıntraabdominal adhesions in rabbits]. FÜ Sağ. Bil. Vet. Derg. [Internet]. 2015 [cited May 22, 2025]; 29(2):75–79. Turkish. Available in: https://goo.su/NLtAQrj
Kiyakli E, Köm M, Eröksüz Y, Baydar E. Ratlarda İntraabdominal Adezyonların Önlenmesinde Karboksimetilselüloz, Meloksikam ve Vitamin E Kombinasyonlarının Etkisi [Effect of combinations of carboxymethycellulose, Meloxicam and vitamin E on preventing ıntraabdominal adhesions in rats]. FÜ Sağ. Bil. Vet. Derg. [Internet]. 2017 [cited May 22, 2025]; 31(3):205–212. Turkish. Available in: https://goo.su/pupzx
Sağliyan A, Aydin HB, Günay C, Durmus AS, Ceribasi S, Polat E. Fluniksin Meglumin ve Amniyon Sıvısının İntraabdominal Adezyonların Önlenmesi Üzerine Etkileri [The effects of Flunixin Meglumine and amnion fluid on the prevention of intraabdominal adhesions]. Erciyes Üniv. Vet. Fak. Derg. [Internet]. 2021; 18(2):75–83. Turkish. doi: https://doi.org/gkqqrb DOI: https://doi.org/10.32707/ercivet.952876
Köm M, Akay I, Polat E, Calik I. Ratlarda İntrabdominal Adezyonların Üzerine Trombositten Zengin Plazma’nın Etkisi [Effect of platelet rich plasma on ıntraabdominal adhesions in rats]. Van Vet. J. [Internet]. 2024; 35(1):1–6. Turkish. doi: https://doi.org/qgct DOI: https://doi.org/10.36483/vanvetj.1385092
Deynez G. Deneysel intra–abdominal adezyon modelinde etkili olabilecek tıbbi bitkiler üzerinde farmakognozik araştırmalar [Pharmacognosic evaluation of medicinal plants that can be effective against surgically–induced intra–abdominal adhesion model]. [dissertation on the Internet]. Ankara (Türkiye): University of Gazi; 2024 [cited May 6, 2025]. 173 p. Turkish. Available in: https://goo.su/LCP0l
Günay C, Sağliyan A, Yaman İ. Ratlarda Deneysel Olarak Oluşturulan İntraabdominal Adezyonların Önlenmesinde Aprotinin İle Metilen Mavisinin Etkinliğinin Karşılaştırılması [Effects of aprotinin and methylene blue in prevention of experimentally performed intraabdominal adhesions in rats]. FÜ Sağlik Bil. Derg. [Internet]. 2005 [cited May 15, 2025]; 19(1):51–55. Turkish. Available in: https://goo.su/grMn6q
Kabalar RB, Şahin ST, Ayhan S. Effect of intra–abdominal boric acid in the experimental adhesion model. Ulus Travma Acil. Cerrahi. Derg. [Internet]. 2024; 30(4):236–241. doi: https://doi.org/qgcv DOI: https://doi.org/10.14744/tjtes.2024.77767
Arslan E. Sıçanlarda oluşturulan deneysel peritoneal adezyon modelinde Seprafilm ve Lovastatin’in karşılaştırılması [Comparison of lovastatin and seprafilm on experimental created peritoneal adhesion model in rats]. [thesis of specialization on the Internet]. Kayseri (Türkiye): University of Erciyes; 2009 [cited May 8, 2025]. 72 p. Turkish. Available in: https://goo.su/YNHD
Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol. Therapeutics [Internet]. 2002; 96(2–3):67–202. doi: https://doi.org/fk38j8 DOI: https://doi.org/10.1016/S0163-7258(02)00298-X
Ünal M, Öztürk O, Selcuk MY, Oruç MA. Propolis – Literatür Ne Diyor? [Propolis – What does the literature say?] Bozok Med J. [Internet]. 2020 [cited May 15, 2025]; 10(2):215–223. Turkish. Available in: https://goo.su/9zonY
Birben B. Postoperatif Adezyonları Önlemede İntraperitoneal Propolis Tedavisinin Etkisi [The effect of propolis treatment to prevent postoperative ı̇ ntraperitoneal adhesions]. [thesis of specialization on the Internet]. Ankara (Türkiye): Başkent Üniversitesi; 2014 [cited May 9, 2025]. 71 p. Turkish. Available in: https://goo.su/bzahEc
Yücel B, Topal E, Akçiçek E, Kösoğlu M. Propolisin İnsan Sağlığına Etkileri [Effects of propolis on human health]. Anadolu. J. AARI. [Internet]. 2014 [cited May 13, 2025]; 24(2):41–49. Turkish. Available in: https://goo.su/BpYqzya
Miyataka H, Nishiki M, Matsumoto H, Fujimoto T, Matsuka M, Satoh T. Evaluation of propolis. I. Evaluation of Brazilian and Chinese propolis by enzymatic and physico–chemical methods. Biol. Pharm. Bull. [Internet]. 1997; 20(5):496–501. doi: https://doi.org/fgz3nc DOI: https://doi.org/10.1248/bpb.20.496
Marquele FD, DI Mambro VM, Georgetti SR,Casagrande R, Valim YM, Fonseca MJ. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J. Pharm. Biomed. Anal. [Internet]. 2005; 39(3– 4):455–462. doi: https://doi.org/c2wdmr DOI: https://doi.org/10.1016/j.jpba.2005.04.004
Celik S, Gorur S, Aslantas O, Erdogan S, Ocak S, Hakverdi S. Caffeic acid phenethyl ester suppresses oxidative stress in Escherichia coli–induced pyelonephritis in rats. Mol. Cell. Biochem. [Internet]. 2007; 297:131–138. doi: https://doi.org/ck9n32 DOI: https://doi.org/10.1007/s11010-006-9337-x
Song YS, Park EH, Hur GM, Ryu YS, Lee YS, Lee JY, Kim YM, Jin C. Caffeic acid phenethyl ester inhibits nitric oxide synthase gene expression and enzyme activity. Cancer Lett. [Internet]. 2002; 175:53–61. doi: https://doi.org/c8gw29 DOI: https://doi.org/10.1016/S0304-3835(01)00787-X
Cikman O, Bulut A, Taysi S. Protective effect of propolis in protecting against radiation-induced oxidative stress in the liver as a distant organ. Sci. Rep. [Internet]. 2024; 14:21766. doi: https://doi.org/qgcw DOI: https://doi.org/10.1038/s41598-024-72344-9
Askari VR, Rahimi VB, Zamani P, Fereydouni N, Rahmanian– Devin P, Sahebkar AH, Rakhshandeh H. Evaluation of the effects of Iranian propolis on the severity of post operational– induced peritoneal adhesion in rats. Biomed. Pharmacother. [Internet]. 2018; 99:346–353. doi: https://doi.org/gc7xx6 DOI: https://doi.org/10.1016/j.biopha.2018.01.068
Nair SK, Bhat IK, Aurora AR. Role of proteolytic enzymes in the prevention of postoperative intraabdominal adhesions. Arch. Surg. [Internet]. 1974; 108(6):849–853. doi: https://doi.org/fpf8nj DOI: https://doi.org/10.1001/archsurg.1974.01350300081019
Celepli S, Kismet K, Kaptanoğlu B,Erel S, Ozer S, Celepli P, Kaygusuz G, Devrim E, Gencay O, Sorkun K, Durak I, Akkuş MA.The effect of oral honey and pollen on postoperative intraabdominal adhesions. Turk. J. Gastroenterol. [Internet]. 2011; 22(1):65–72 .doi: https://doi.org/qgcx DOI: https://doi.org/10.4318/tjg.2011.0159
Placer ZA, Cushman L, Johnson BC. Estimation of products of lipid peroxidation in biological fluids. Anal. Biochem. [Internet]. 1966; 16:359–364. doi: https://doi.org/b96rpj DOI: https://doi.org/10.1016/0003-2697(66)90167-9
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. [Internet]. 1961; 7:88–95. doi: https://doi.org/fwdkkz DOI: https://doi.org/10.1016/0006-2952(61)90145-9
Aebi H. Catalase in vitro. Methods Enzymol. [Internet]. 1984; 105:121–126. doi: https://doi.org/dnf7v9 DOI: https://doi.org/10.1016/S0076-6879(84)05016-3
Beutler E. Red cell metabolism. A manual of biochemical methods. 2nd ed. New York (USA): Grune and Starton; 1984. 160 p.
Sun Y, Oberly LW, Ying LA. Simple method for clinical assay of superoxide dismutase. Clin. Chem. [Internet]. 1988; 34(3):497–500. Available in: https://goo.su/Y6UMm DOI: https://doi.org/10.1093/clinchem/34.3.497
Frankel S, Reitman S, Sonnen AC. A textbook on laboratory procedure and their interpretation. In: Gradwohl RBH, editor. Gradwohl’s Clinical Laboratory Methods and Diagnosis. St. Louis (USA): The CV Mosby Company; 1970. 403–404 p.
Sumbuloglu K, Sumbuloglu V. Biostatistics. 19th. ed. Ankara (Türkiye): Hatiboglu Yayinevi. 2019; p. 100–179.
Kraemer B, Wallwiener C, Rajab TK,Brochhausen C, Wallwiener M, Rothmund R. Standardised models for inducing experimental peritoneal adhesions in female rats. BioMed. Res. Int. [Internet]. 2014; 435056:1–8. doi: https://doi.org/gb9knh DOI: https://doi.org/10.1155/2014/435056
Nian H, Pu Z, Li Z, Zhong P, Ma S, Li J. Establishment and evaluation of a stable and reliable rat model of peritoneal adhesions. Surgery [Internet]. 2024; 176(4):1256–1262. doi: https://doi.org/qgcz DOI: https://doi.org/10.1016/j.surg.2024.06.034
Gümüşgerdanli AC. Ratlarda Sistemik Rapamisin Uygulamasının Postoperatif Peritoneal Adezyonlar Üzerine Etkisi [Effects of systemic rapamycine administration on postoperative peritoneal adhesions in rats]. [thesis of specialization on the Internet]. Izmir (Türkiye): University of Dokuz Eylul; 2011 [cited May 10, 2025]. 50 p. Turkish. Available in: https://goo.su/Mpoc
Diken Allahverdi T, Allahverdi E, Yayla S,Deprem T, Merhan O, Vural S, Sülü B, Günerhan Y, Köksal N. Effects of alpha lipoic acid on intra–abdominal adhesion: an experimental study in a rat model. Ulus Travma Acil. Cerrahi. Derg. [Internet]. 2015; 21(1):9–14.doi: https://doi.org/f7cf8q DOI: https://doi.org/10.5505/tjtes.2015.15985