La nicotinamida ribósido, un precursor y suplemento de NAD, reduce el daño hepático causado por la sepsis
Resumen
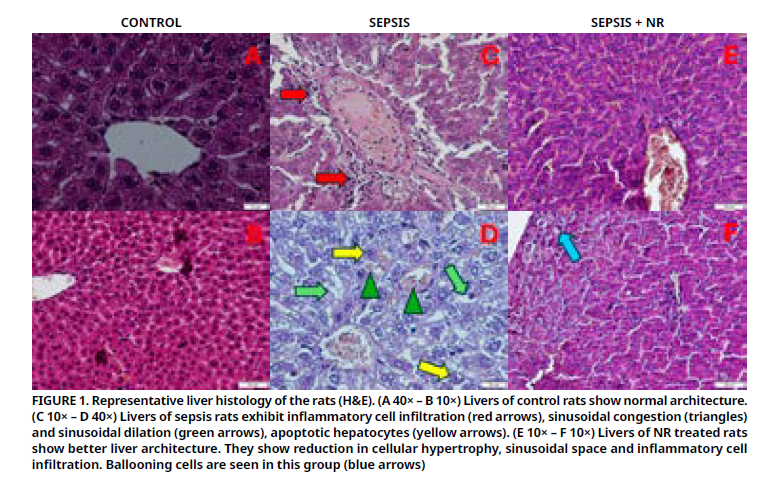
La sepsis conduce a insuficiencia hepática y finalmente, a la muerte. El suplemento ribósido de nicotinamida es un antioxidante y precursor del dinucleótido de nicotinamida y adenina. Este estudio tuvo como objetivo evaluar el efecto el efecto terapéutico del ribósido de nicotinamida sobre la lesión hepática en un modelo de sepsis en ratas inducida por ligadura y perforación cecal. Se dividieron 21 ratas en 3 grupos: control, sepsis y sepsis + ribósido de nicotinamida. Se realizó ligadura y perforación cecal en los grupos sepsis y sepsis + ribósido de nicotinamida. Al grupo sepsis + ribósido de nicotinamida se le administró ribósido de nicotinamida por vía oral (200 mg·kg-1) 1 hora (h) antes y 12 h después de la ligadura y perforación cecal. Veinticuatro horas después de la ligadura y perforación cecal, se sacrificaron las ratas. Se analizaron parámetros histopatológicos y bioquímicos. El tratamiento con ribósido de nicotinamida resultó en una disminución de los niveles elevados de aspartato aminotransferasa, alanina aminotransferasa, creatina y ceruloplasmina. Los niveles séricos de albúmina, calcio y amilasa disminuyeron en el grupo con sepsis y aumentaron en el grupo con sepsis y control (P<0,05). Sin embargo, no se observaron diferencias significativas en los niveles de ácido úrico, sodio, magnesio y cloruro entre los grupos. La estructura hepática dañada por la sepsis mejoró histológicamente. Los niveles de superóxido dismutasa fueron bajos en el grupo con sepsis, pero aumentaron significativamente en el grupo con sepsis y control (P<0,05). Los niveles de malondialdehído fueron altos en el grupo con sepsis, pero aumentaron en el grupo con sepsis y control (P<0,05). El ribósido de nicotinamida mitigó el daño tisular hepático en ratas sépticas. Corrigió el desequilibrio oxidante-antioxidante y algunos parámetros séricos. Esto podría ser evidencia del efecto beneficioso del ribósido de nicotinamida en el hígado. No obstante, se deben realizar nuevas investigaciones para comprender mejor el mecanismo.
Descargas
Citas
Dkhil MA, Al-Quraishy S, Moneim AEA. Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via anti-oxidant and anti-inflammatory effects. Inflammopharmacology [Internet]. 2018; 26:779–791. doi: https://doi.org/qq45
Woznica EA, Inglot M, Woznica RK, Lysenko L. Liver dysfunction in sepsis. Adv. Clin. Exp. Med. [Internet]. 2018; 27(4):547–551. doi: https://doi.org/ghn5qm
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). J. Am. Med. Assoc. [Internet]. 2016; 315(8):801–810. doi: https://doi.org/gdrcdh
Cao T, Ni R, Ding W, Ji X, Fan GC, Zhang Z, Peng T. Nicotinamide mononucleotide as a therapeutic agent to alleviate multi-organ failure in sepsis. J. Transl. Med. [Internet]. 2023; 21:883. doi: https://doi.org/qq47
Shi H, Han W, Shi H, Ren F, Chen D, Chen Y, Duan Z. Augmenter of liver regeneration protects against carbon tetrachloride-induced liver injury by promoting autophagy in mice. Oncotarget [Internet]. 2017; 8:12637–12648. doi: https://doi.org/f9k6sb
Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J. Glob. Antimicrob. Resist. [Internet]. 2017; 10:204–212. doi: https://doi.org/gg328p
Hong G, Zheng D, Zhang L, Ni R, Wang G, Fan GC, Lu Z, Peng T. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic. Biol. Med. [Internet]. 2018; 123:125–137. doi: https://doi.org/gdtn4r
Ye M, Zhao Y, Wang Y, Xie R, Tong Y, Sauer JD, Gong S. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat. Nanotechnol. [Internet]. 2022; 17:880–890. doi: https://doi.org/gqbx88
Trammell SAJ, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J. Nutr. [Internet]. 2016; 146(5):957–963. doi: https://doi.org/f8kgjq
Kang H, Park YK, Lee JY. Nicotinamide riboside attenuates inflammation and oxidative stress by activating sirtuin-1 in alcohol-stimulated macrophages. Lab. Investig. [Internet]. 2021; 101(9):1225–1237. doi: https://doi.org/gqpxj8
Liu A, Wang W, Fang H, Yang Y, Jiang X, Liu S, Hu J, Hu Q, Dahmen U, Dirsch O. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur. J. Pharmacol. [Internet]. 2015; 748:45–53. doi: https://doi.org/f6x8q3
Hamity MV, White SR, Blum C, Gibson-Corley KN, Hammond DL. Nicotinamide riboside relieves paclitaxel-induced peripheral neuropathy and enhances suppression of tumor growth in tumor-bearing rats. Pain [Internet]. 2020; 161(10):2364–2375. doi: https://doi.org/qq49
Yener MD, Çolak T, Özsoy ÖD, Eraldemir FC. Alterations in catalase, superoxide dismutase, glutathione peroxidase and malondialdehyde levels in serum and liver tissue under stress conditions. Istanb. Tip Fak. Derg. [Internet]. 2024; 87(2):145–152. doi: https://doi.org/qq5b
Muftuoglu MAT, Aktekin A, Ozdemir NC, Saglam A. Liver injury in sepsis and abdominal compartment syndrome in rats. Surg. Today [Internet]. 2006; 36:519–524. doi: https://doi.org/d6spxt
Zhu Z, Chambers S, Bhatia M. Suppressing the substance P-NK1R signalling protects mice against sepsis-associated acute inflammatory injury and ferroptosis in the liver and lungs. Antioxidants [Internet]. 2024; 13(3):300. doi: https://doi.org/qq5c
Cerrah S, Cadirci E, Okcu N, Deveci O. The determination of the protective role of sildenafil administration in rats with sepsis-induced liver injury. Turk. J. Trauma Emerg. Surg. [Internet]. 2023; 29(2):133–139. doi: https://doi.org/qq5d
Liu X, Yang X, Han L, Ye F, Liu M, Fan W, Zhang K, Kong Y, Zhang J, Shi L, Chen Y, Zhang X, Lin S. Pterostilbene alleviates polymicrobial sepsis-induced liver injury: possible role of SIRT1 signaling. Int. Immunopharmacol. [Internet]. 2017; 49:50–59. doi: https://doi.org/gbp58c
Peng X, Dai C, Liu Q, Li J, Qiu J. Curcumin attenuates carbon tetrachloride-induced acute liver injury in mice via modulation of the Nrf2/HO-1 and TGF-β1/Smad3 pathway. Molecules [Internet]. 2018; 23(1):215. doi: https://doi.org/g77c74
Sakhuja P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J. Gastroenterol. [Internet]. 2014; 20(44):16474–16479. doi: https://doi.org/f6r9b4
Turck D, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Kearney J, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M. Safety of nicotinamide riboside chloride as a novel food and bioavailability of nicotinamide from this source, in the context of Directive 2002/46/EC. EFSA J. [Internet]. 2019; 17(8):e05775. doi: https://doi.org/qq5r
Bonilla DA, Kreider RB, Stout JR, Forero DA, Kerksick CM, Roberts MD, Rawson ES. Metabolic basis of creatine in health and disease: a bioinformatics-assisted review. Nutrients [Internet]. 2021; 13(4):1238. doi: https://doi.org/gs8wg4
Nedel W, Deutschendorf C, Portela LVC. Sepsis-induced mitochondrial dysfunction: a narrative review. World J. Crit. Care Med. [Internet]. 2023; 12(3):139–152. doi: https://doi.org/qq5t
Casciola R, Leoni L, Cuffari B, Pecchini M, Menozzi R, Colecchia A, Ravaioli F. Creatine supplementation to improve sarcopenia in chronic liver disease: facts and perspectives. Nutrients [Internet]. 2023; 15(4):863. doi: https://doi.org/qq5s
Xu L, Han G. Research progress in pharmacokinetics of phosphocreatine, a cardioprotective agent with dual antiplatelet activity. Ann. Vasc. Med. Res. [Internet]. 2023; 10(4):1173. doi: https://doi.org/qq5v
Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. [Internet]. 2018; 27(3):513–528. doi: https://doi.org/gc7pkf
Klimova N, Long A, Kristian T. Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice. J. Neurosci. Res. [Internet]. 2019; 97(8):975–990. doi: https://doi.org/ggts79
Arslan NP, Akpinar Z, Aybek H, Doymus M, Asilkan-Kaldik G, Esim N, Taskin M. NAD+ precursors mitigate reproductive defects: limitations and possible solutions. Reprod. Toxicol. [Internet]. 2025; 138:109067. doi: https://doi.org/qq5w
Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. [Internet]. 2020; 5:227. doi: https://doi.org/ghrs66
Chaari A, Hakim KA, Rashed N, Bousselmi K, Kauts V, Etman M, Casey WF. Factors associated with increased pancreatic enzymes in septic patients: a prospective study. J. Intensive Care [Internet]. 2017; 5:44. doi: https://doi.org/qq5x
Tonai K, Katayama S, Koyama K, Imahase H, Nunomiya S. Association between hypomagnesemia and serum lactate levels in patients with sepsis. J. Anesth. Analg. Crit. Care [Internet]. 2024; 4:23. doi: https://doi.org/qq5z
Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia is associated with acute kidney injury in severe sepsis. Crit. Care [Internet]. 2016; 20:315. doi: https://doi.org/gjvkcb
Han Y, Duan J, Chen M, Huang S, Zhang B, Wang Y, Liu J, Li X, Yu W. Relationship between serum sodium level and sepsis-induced coagulopathy. Front. Med. [Internet]. 2023; 10:1324369. doi: https://doi.org/qq52
He W, Huang L, Luo H, Zang Y, An Y, Zhang W. Hypocalcemia in sepsis: subcellular distribution of Ca2+ in septic rats and LPS·TNF-1-α-treated HUVECs. J. Infect. Dev. Ctries. [Internet]. 2020; 14:908–917. doi: https://doi.org/gpg7c9
Saravi B, Goebel U, Hassenzahl LO, Jung C, David S, Feldheiser A, Stopfkuchen-Evans M, Wollborn J. Capillary leak and endothelial permeability in critically ill patients: a current overview. Intensive Care Med. Exp. [Internet]. 2023; 11:96. doi: https://doi.org/qq53
Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J. Parenter. Enter. Nutr. [Internet]. 2019; 43(2):181–193. doi: https://doi.org/gk45fg
Hayashi N, Yamaguchi S, Rodenburg F, Wong SY, Ujimoto K, Miki T, Iba T. Multiple biomarkers of sepsis identified by time-lapse proteomics. PLoS One [Internet]. 2019; 14(9):e0222403. doi: https://doi.org/grkr9p
Linder MC. Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics [Internet]. 2016; 8(9):887–905. doi: https://doi.org/gpdntz
Neşelioğlu S, Oğuz EF, Erel Ö. Development of a new Colorimetric, Kinetic and automated ceruloplasmin ferroxidase activity measurement method. Antioxidants [Internet]. 2022; 11(11):2187. doi: https://doi.org/qq54
Derechos de autor 2026 Zeliha Yetim

Esta obra está bajo licencia internacional Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0.


















